
Commonly used bearing steel is divided into four categories: high-carbon chromium bearing steel, carburized bearing steel, stainless bearing steel and high temperature bearing steel. Or according to chemical composition, performance, use of processing technology and use, it is divided into fully quenched bearing steel, carburized bearing steel, stainless bearing steel and high temperature bearing steel.
Among them, the high-carbon chromium bearing steel GCr15 is the largest bearing steel, accounting for more than 80% of the bearing steel consumption, the carbon content is about 1%, the chromium content is about 1.5%, usually, if not specifically specified, the bearing steel default refers to GCr15. This kind of bearing steel is mostly used for small and medium-sized rolling bearings, medium and large bearings with large wall thickness, and GCr15SiMn material is selected to improve hardenability.
Bearing steel is mainly used to manufacture rolling elements and bearing rings. It has high and uniform hardness and wear resistance, as well as a high elastic limit, bearing steel on the chemical composition uniformity, non-metallic inclusions content and distribution, carbide distribution and other requirements are extremely strict, is one of the most stringent steel production requirements.
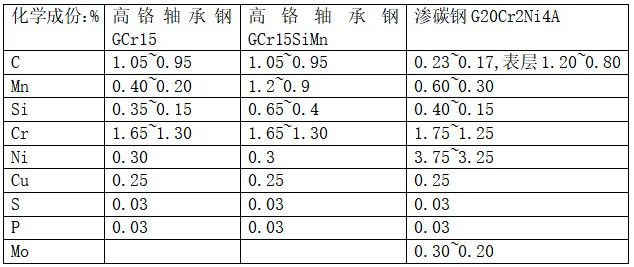
The main element contained in the bearing steel is iron, trace beneficial elements are carbon, chromium, manganese, molybdenum, nickel, silicon, tungsten, vanadium, etc., trace harmful elements mainly refer to phosphorus, sulfur, P, S content is not greater than 0.025%, oxygen content also has control requirements.
1. The role of carbon:
The role of carbon in steel is crucial, if there is no carbon containing iron in chemistry is called pure iron, his hardness is extremely low, almost as seen in sterling silver with a slight bite is a pit. Such iron is not called the steel on the market, and it is only possible to use such pure iron in chemical laboratories. If steel is the bone of the construction industry, then carbon is the bone of steel. It can be seen that the importance of carbon is irreplaceable by other elements.
Bearing ring and rolling body through different heat treatment processing technology, so that the bearing internal has different carbide crystal structure, the surface reaches high hardness and wear resistance, usually bearing ring surface hardness HRC62~58, rolling body surface HRC63~59.
2. The role of chromium:
Chromium can make bearing steel after quenching, tempering to obtain a stable carbide internal structure, with good comprehensive mechanical properties, in carburized steel can also form chromium carbide, thereby improving the wear resistance of the surface of the material. Bearing surface grinding, easy to obtain a high surface finish, chromium element will also improve the bearing steel oxidation resistance, corrosion resistance.
3. The role of silicon:
Silicon can improve hardenability, silicon can be dissolved in ferrite and austenite to improve the hardness and strength of steel, its role is second only to phosphorus, stronger than manganese, nickel, chromium, tungsten, molybdenum and vanadium and other elements. However, when the silicon content exceeds 3%, the plasticity and toughness of the steel will be significantly reduced. Silicon can improve the elastic limit, yield strength and yield ratio (σs/σb), and fatigue strength and fatigue ratio (σ-1/σb) of steel.
Silicon can reduce the density, thermal conductivity and electrical conductivity of steel. It can promote ferrite grain coarsening and reduce coercivity. The addition of silicon has the tendency to reduce the anisotropy of the crystal, making it easy to magnetize, reduce the magnetic resistance, and facilitate the processing of bearing parts. Silicon can reduce the welding performance of the steel, because the affinity of silicon with oxygen is stronger than iron, it is easy to generate low melting point silicate during welding, increase the fluidity of slag and molten metal, cause sputtering phenomenon, and affect the quality of the weld. Bearing steel cannot be welded.
4. The role of manganese:
Manganese is a good deoxidizer and desulfurizer, industrial steel contains a certain amount of manganese, manganese soluble in ferrite and austenite, can expand the austenite region, so that the critical temperature increases, manganese greatly reduces the martensitic transition temperature of steel (its role is second only to carbon) and the speed of phase change in steel, improve the hardenability of steel, but will increase the residual austenite content, The tempered structure of the steel is uniform and refined, and the carbides in the carburizing layer are avoided to accumulate into blocks. However, it increases the overheating sensitivity and tempering brittleness of steel, and manganese is a weak carbide forming element.
The effect of manganese on strengthening ferrite or austenite is not as good as that of carbon, phosphorus and silicon, and it has no effect on the ductility while increasing the strength. Because the pearlite is refined, the strength of low carbon and medium carbon pearlite steel is significantly improved, and the ductility is reduced.
When manganese content is high, the oxidation resistance of steel decreases, and MnS with high melting point is formed with sulfur in steel, which avoids the formation of FeS film on the grain boundary, eliminates the hot brittleness of steel, and improves the hot working performance. The deformation resistance of high manganese austenitic steel is large, and the columnar crystallization in the ingot is obvious, and it is easy to crack during forging and rolling. Due to the improvement of hardenability and the reduction of martensitic transition temperature, the welding performance is adversely affected. Manganese has a tendency to increase the grain coarsing of steel, and if the cooling after smelting and forging is improper, the steel is easy to produce white spots.
5. The role of nickel:
Nickel and iron can be infinite solid solution, nickel expands the austenite region of iron, is the main alloying element to form and stabilize austenite, nickel and carbon do not form carbides, reduce the critical transition temperature, reduce the diffusion rate of the elements in steel, improve hardenability, reduce the carbon content of eutoid pearlite, its role is second only to nitrogen and stronger than manganese. The effect in reducing the martensitic transition temperature is about half that of manganese.
Strengthen ferrite and refine and increase pearlite, improve the strength of steel, the plastic effect of steel is less, common nickel-containing steel products as shown in the figure, carburized steel has a higher nickel content, improve the toughness and plasticity of bearing steel, can significantly improve the bearing fatigue resistance, impact toughness performance, nickel-containing steel has a certain corrosion resistance to acid, alkali and atmosphere.