
2 Iron-carbon alloy
2.1 Iron-carbon phase diagram
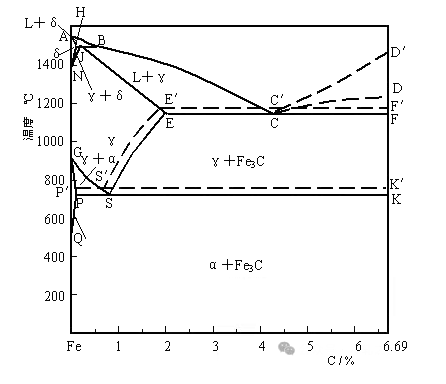
A1:PSK
A3:GS
Am:SE
2.1.1Basic phase
2.1.1.1Ferrite
The interstitial solid solution formed when carbon dissolves in α -Fe is called ferrite, denoted by the symbol F. The solubility of carbon in α -Fe is relatively low. It can reach a maximum of 0.0218% at 727℃ and is only about 0.0008% at room temperature. Because the carbon content of ferrite is very low, its structure and properties are extremely similar to those of pure iron, and it has very good plasticity and toughness, with extremely low strength and hardness.
2.1. 1.2Austenite
The interstitial solid solution formed when carbon dissolves in γ-Fe is called austenite, which is commonly represented by the symbol A. The carbon capacity of austenite is relatively high, reaching the highest at 1148℃, which can reach 2.11%. It is 0.77% at 727℃. Austenite has relatively low hardness and high plasticity, making it easy for pressure processing. During the hot rolling process, it is in austenite.
2.1. 1.3Cementite
The compound Fe3C formed by iron and carbon is called cementite. The carbon content of cementite is approximately 6.69%. Cementite has a very high hardness, while its plasticity and toughness are almost zero, making it a hard and brittle phase.
2.1.2The application of iron-carbon phase diagrams
2.1.2.1The most reasonable basis for choosing materials
Through the analysis of the iron-carbon phase diagram, the variation laws of the alloy's microstructure and properties with composition can be understood. Thus, materials can be selected based on the working conditions and performance requirements of the parts. If materials with high plasticity and toughness are required, steel with a low carbon content (0.10-0.25%) should be selected. For materials with good strength, plasticity and toughness, hypoeutectoid steel with medium carbon content (0.25-0.6%) can be selected. For materials with good wear resistance and high hardness, steel with a high carbon content (0.60-1.40%) should be selected.
2.1.2.2As the basis for formulating the thermal processing technology.
1、In terms of hot rolling. At room temperature, the structure of steel is a two-phase mixture with poor plasticity and difficult deformation. Only when it is heated to the single-phase austenite state can it have better strength and plasticity. Therefore, the general initial rolling temperature is 100-200 degrees Celsius below the solid phase line, while the final rolling temperature is slightly higher than A3 for hypoeutectoid steel and slightly higher than A1 for hypereutectoid steel.
2、Annealing process
2.2 The influence of common elements on the properties of steel
2.2.1carbon
With the increase of carbon content, the hardness of steel rises sharply, while its plasticity and toughness decrease. For the ultimate strength (tensile strength), within the range of hypoeutectoid steel, as the carbon content increases, the strength continuously rises due to the increase in pearlescent volume. After exceeding the eutectoid composition, secondary cementite begins to appear on the microstructure. When the carbon content is lower than 0.9%, the volume of secondary cementite is small and it cannot form a network, so the strength continues to increase; while when the carbon content is greater than 0.9%, the network of secondary cementite tends to be complete, so the strength significantly decreases.
2.2.2sulfur
Sulfur has an extremely low solubility in iron and forms the compound FeS with iron (melting point 1190℃). FeS and Fe can form a low-melting-point eutectic (melting point 985℃), which is distributed along the grain boundaries. During hot pressure processing, due to the easy melting of the eutectic of FeS and Fe, cracking occurs. This phenomenon is called hot brittleness.
To eliminate the harmful effects of sulfur, a certain amount of manganese should be contained in the steel. This is because the affinity between manganese and sulfur is greater than that between iron and sulfur. Manganese can preferently form high-melting-point (1620) MnS with sulfur, effectively preventing hot cracking. The content of sulfur is generally limited to below 0.055%.
2.2.3phosphorus
Even if the phosphorus content in steel is only a few thousandths, the brittleness of the steel will increase due to the precipitation of the brittle compound Fe3P, especially at low temperatures. The phenomenon where the toughness of an alloy drops sharply when the temperature drops is called cold brittleness.
Phosphorus not only causes cold brittleness but also reduces the weldability of steel. When its content is too high, cracks are prone to occur in the weld seam. Therefore, phosphorus is also regarded as a harmful impurity in steel, and its content in steel is generally controlled below 0.045%. However, phosphorus can enhance the corrosion resistance of steel.
2.2.4manganese
Manganese has a certain deoxidizing capacity, which can eliminate the oxidizing characteristics in steel and significantly improve the quality of steel. It can form the compound MnS with S, reducing the harmful effects of sulfur. It can also dissolve in ferrite to form a displacement solid solution, thereby strengthening the steel. Manganese can also increase the relative amount of pearlite and refine the tissue. However, when manganese exists as a small amount of impurity, its influence on the mechanical properties of carbon steel is not significant. Therefore, manganese is a beneficial element.
2.2.5silicon
The role of silicon is similar to that of manganese. Its deoxidation capacity is even stronger than that of manganese. However, it increases the strength, hardness and elasticity of steel while reducing its plasticity and toughness. Silicon as an impurity in steel is generally less than 0.5%
2.2.6hydrogen
Hydrogen is a harmful element in steel, which manifests in two aspects:
1)Hydrogen dissolving in steel reduces its plasticity and toughness, causing what is known as hydrogen embrittlement. Hydrogen embrittlement neither occurs at very low temperatures nor at high temperatures. The phenomenon of hydrogen embrittlement only occurs within a specific temperature range of -100 to 100℃.
2) Hydrogen has no significant effect on the plasticity of steel during hot working, as it partially precipitates from the steel when heated to around 1000℃. However, for steel grades with a relatively high hydrogen content, if they cool down rapidly after hot working, the hydrogen atoms precipitated from the solid solution will not have enough time to diffuse to the steel surface, but will concentrate at the grain boundaries and micropores to form hydrogen molecules and generate considerable stress. Under the combined action of microstructure stress, temperature stress and internal stress caused by hydrogen precipitation, fine cracks and white spots will appear in the steel. This phenomenon is particularly serious in alloy steel.
2.2.7nitrogen
At 591℃, the solubility of nitrogen in ferrite is the highest, approximately 0.1%, but it decreases to below 0.001% at room temperature. When steel with a relatively high nitrogen content is cooled rapidly from high temperatures, the nitrogen in ferrite becomes supersaturated. At room temperature or slightly higher temperatures, nitrogen will gradually precipitate in the form of Fe4N, resulting in an increase in the strength and hardness of the steel, while its plasticity and toughness are significantly reduced, making the steel brittle. This phenomenon is called aging brittleness. The solution is to add a sufficient amount of aluminium to the steel, so that it can combine with nitrogen to form AlN during the slow cooling process after hot rolling (700-800℃), in addition to combining with oxygen. This can weaken or eliminate the aging phenomenon that occurs at room temperature.
In addition, the use of dispersed ALN can prevent the growth of austenite in steel during heating, thereby obtaining fine-grained steel.
2.2.8Other
Cr
1. In the range of low alloy, it has a great strengthening effect on steel, improving strength, hardness and wear resistance
2. Reduce the critical cooling rate of steel and enhance its hardenability
3. Enhance the heat resistance of steel
4. Within the high alloy range, it enables the steel to have the corrosion resistance to strong oxidizing acids and other corrosive media
Mo
1.Strengthen ferrite to enhance the strength and hardness of steel
2. Reduce the critical cooling rate of steel and enhance its hardenability
3. Enhance the heat resistance and high-temperature strength of steel
Ni
1. Improve the strength of steel, without reducing its plasticity, improve the low temperature toughness of steel
2. Reduce the critical cooling rate of steel and enhance its hardenability
3. Expanding the austenite region is an effective element for austenitization
4. It has a certain degree of corrosion resistance by itself and shows good resistance to some reducing acids
Al
1. Good deoxygenation in steelmaking
2. Refine the grains of steel and enhance its strength
3. Enhance the oxidation resistance of steel and improve the corrosion resistance of stainless steel to strong oxidizing acids